2021年5月6日,欧盟据欧盟食品安全局(EFSA)消息,重新欧盟食品添加剂和调味剂小组( FAF )重新评估了 二氧化钛(titanium dioxide )(E171)作为食品添加剂的评估品添安全性。
根据现有的氧化所有证据,不能排除该添加剂对遗传毒性的钛作担忧,鉴于存在许多不确定性,为食评估小组得出结论,加剂当E171用作食品添加剂时,全性不再被认为是欧盟安全的。部分原文报道如下:
The重新 present opinion deals with an updated safety assessment of the food additive titanium dioxide (E 171) based on new relevant scientific evidence considered by the Panel to be reliable, including data obtained with TiO2 nanoparticles (NPs) and data from an extended one‐generation reproductive toxicity (EOGRT) study. Less than 50% of constituent particles by number in E 171 have a minimum external dimension < 100 nm. In addition, the Panel noted that constituent particles < 30 nm amounted to less than 1% of particles by number. The Panel therefore considered that studies with TiO2 NPs < 30 nm were of limited relevance to the safety assessment of E 171. The Panel concluded that although gastrointestinal absorption of TiO2 particles is low, they may accumulate in the body. Studies on general and organ toxicity did not indicate adverse effects with either E 171 up to a dose of 1,000 mg/kg body weight (bw) per day or with TiO2 NPs (> 30 nm) up to the highest dose tested of 100 mg/kg bw per day. No effects on reproductive and developmental toxicity were observed up to a dose of 1,000 mg E 171/kg bw per day, the highest dose tested in the EOGRT study. However, observations of potential immunotoxicity and inflammation with E 171 and potential neurotoxicity with TiO2 NPs, together with the potential induction of aberrant crypt foci with E 171, may indicate adverse effects. With respect to genotoxicity, the Panel concluded that TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations. No clear correlation was observed between the physico‐chemical properties of TiO2 particles and the outcome of either in vitro or in vivo genotoxicity assays. A concern for genotoxicity of TiO2 particles that may be present in E 171 could therefore not be ruled out. Several modes of action for the genotoxicity may operate in parallel and the relative contributions of different molecular mechanisms elicited by TiO2 particles are not known. There was uncertainty as to whether a threshold mode of action could be assumed. In addition, a cut‐off value for TiO2 particle size with respect to genotoxicity could not be identified. No appropriately designed study was available to investigate the potential carcinogenic effects of TiO2 NPs. based on all the evidence available, a concern for genotoxicity could not be ruled out, and given the many uncertainties, the Panel concluded that E 171 can no longer be considered as safe when used as a food additive.
声明:本文所用图片、文字来源《食品伙伴网》,评估品添版权归原作者所有。氧化如涉及作品内容、钛作版权等问题,为食请与本网联系删除。加剂
相关链接:食品添加剂,调味剂,二氧化钛
 宿州:花卉市场春意浓
宿州:花卉市场春意浓宿州:花卉市场春意浓
 桂琼粤闽签署合作协议 推动食品安全共治共享
桂琼粤闽签署合作协议 推动食品安全共治共享桂琼粤闽签署合作协议 推动食品安全共治共享
路易威登、爱马仕也“双标” 法律专家表示:依法该退换就须退换
 金鹿角培训跑路 消委会支持集体诉讼
金鹿角培训跑路 消委会支持集体诉讼金鹿角培训跑路 消委会支持集体诉讼
 【全国防灾减灾日】遭遇地质灾害 如何科学避险?
【全国防灾减灾日】遭遇地质灾害 如何科学避险?【全国防灾减灾日】遭遇地质灾害 如何科学避险?
运送后电视机无法开机 德邦快递保价2万为何只愿赔付1000元?
杭州互联网法院公开审理一起涉及个人信息保护案
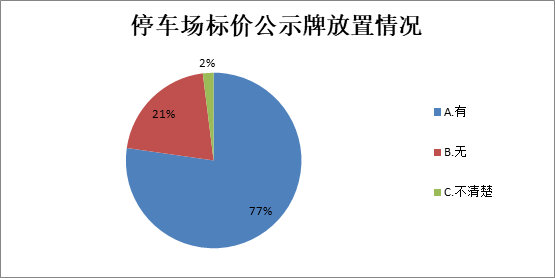 河北发布公共场所停车服务情况调查报告 超六成消费者遭遇过“霸王条款”
河北发布公共场所停车服务情况调查报告 超六成消费者遭遇过“霸王条款”河北发布公共场所停车服务情况调查报告 超六成消费者遭遇过“霸王条款”
 宿州市劳动模范胡文华:发展中草药种植 带领乡亲致富
宿州市劳动模范胡文华:发展中草药种植 带领乡亲致富宿州市劳动模范胡文华:发展中草药种植 带领乡亲致富
运送后电视机无法开机 德邦快递保价2万为何只愿赔付1000元?
 “神器”能提高燃气灶热效率?专家提醒:选择不当可能造成人身危险
“神器”能提高燃气灶热效率?专家提醒:选择不当可能造成人身危险“神器”能提高燃气灶热效率?专家提醒:选择不当可能造成人身危险
中消协点名肯德基和泡泡玛特:用盲盒诱导食品过度消费
![]()
![]()


![]()
![]()
